
ChemWeb | ChemSketch | Moodle | TPK
Kevlar
Kevlar is synthesised from the monomers 1,4-phenylene-diamine (para-phenylenediamine) and terephthaloyl chloride in condensation reaction yielding hydrochloric acid as a byproduct. The result is a liquid-crystalline behaviour and mechanical drawing orienting the polymer chains in the fibre's direction.
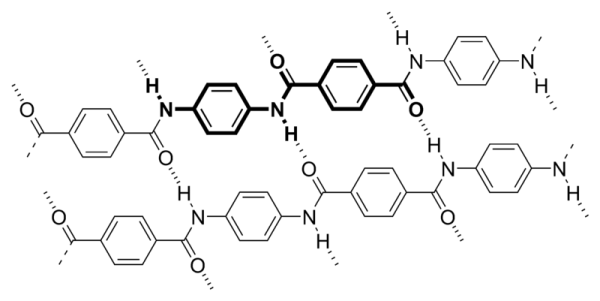
Fibers of Kevlar consist of long molecular chains produced from PPTA (poly-paraphenylene terephthalamide). There are many inter-chain bonds making the material extremely strong. Kevlar derives part of its high strength from inter-molecular hydrogen bonds formed between the carbonyl groups and protons on neighboring polymer chains and the partial pi stacking of the benzenoid aromatic stacking interactions between stacked strands.
Use the mouse to rotate the molecules. With the other mouse button you'll get the menu. The middle button zooms.
More about Jmol is the web: http://jmol.sourceforge.net
A list of all molecule examples in these pages.