Tore Gustafsson
March 23, 1999
Introduction
The pH-control project at the Process Control Laboratory started back in 1975 with a 24-h record of the pH value of a wastewater tank of a chlorine plant. The record revealed an unstable control for one third of the day and a lazy control for the rest of the day.
Modeling and conventional control
The dynamics of stirred reactors with fast acid-base reactions was modeled
by using the concept of reaction invariants [1], which resulted in general
dynamic models for pH involving fast acid-base reactions [2]. Proper mathematical
models for the dynamics of pH was earlier presented by Mellichamp et al.
[3], in contrast to the ad hoc models which flourished at that time (and
later) [4].
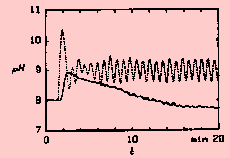
| Figure 1. Measured pH in a pH control tank is oscillating about the set value at pH 9, while the measured pH value of a subsequent settling tank decreases towards 7.7 [5]. |

Some fundamentals in pH control [5], [6], [8]:
The solution to time-varying buffering is adaptive control. Adaptive control can be
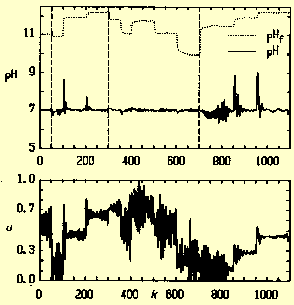
| Figure 2. Experimental adaptive nonlinear pH control of a system with severe variations in buffering [6], [7]. The experimental equipment consists of a controlled stirred tank with a subsequent settling tank. At k = 50 carbonate buffering is reduced to 1/6 of original concentration, at k = 300 buffering is restored to original level and at k = 700 carbonate concentration is reduced to zero mol/l. Additional load changes (strong base concentration) are introduced at additional time instants. Nomenclature: pHf = pH of feed to the stirred tank, pH = controlled pH measured at the outlet of the stirred tank, u = normalized control signal, k = discrete time (sampling instant). |
This controller is compared to adaptive control with linear feedback in reference [7].
Slow acid-base reactions
Acid-base reactions between components in water solution are normally
extremely fast, that is practically in equilibrium at any time. This is
the normal presumption for dynamic models. The standard presumption is,
however, not fulfilled if the reactions involve species, which are present
in solid forms. This is in fact a common case. The characteristics of a
neutralization process can drastically be changed if solid salts are present
[8]. A drastic example of the influence of precipitants is shown in Figure
3. A dynamic model must involve the speed of precipitation and dissolution,
which, however, are not easy to characterize [8].
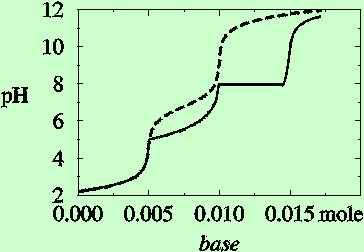
| Figure 3. Titration curves for titration of 0.01 M H3PO4 with NaOH (dashed line) and with Ca(OH)2 (solid line) [8]. Formation of solids deflect the titration curve in the latter case, giving a plateau at pH 8, where the pH of the solution does not change when base is added. |
Robust control
The dynamic model for slow acid-base reactions is a good testbench for multimodel robust control for nonlinear plants. A project on robust pH Control was done in co-operation with the project on Parametric Optimal Robust Control, giving a realistic object for implementation of robust multimodel control in a nonlinear process-environment [9].
The future
What is relevant in pH-control research? Many severe problems in pH
control are solved by a careful choice of equipment (e.g. avoiding dead
times) and conventional linear control. Systems with severe and fast variations
in buffering might need adaptive control. A serious basis for adaptive
control is the method developed in references [2] and [7]. This method
is, however, developed for slowly changing buffering. In a system with
fast variations in buffering this adaptive controller will start to oscillate.
For general use the controller ought to be robustified in order to avoid
oscillations, but it should also be made more robust for increasing buffering,
that is able to swiftly react on decreasing process gains.
References
[1] Asbjørnsen, O.A., Reaction Invariants in the Control of Continuous Chemical Reactors, Chem. Engng Sci. 27 (1972), 709-717.
[2] Gustafsson, T.K. and K.V. Waller, Dynamic Modeling and Reaction Invariant Control of pH, Chem. Engng Sci. 38 (1983), 389-398.
[3] Mellichamp, D.A., D.R. Coughanowr and L.B. Koppel, Characterization and Gain Identification of Time Varying Flow Processes, AIChE J. 12 (1966), 75-82.
[4] Gustafsson, T.K., Calculation of the pH value of a mixture of solutions - an illustration of the use of chemical reaction invariants, Chem. Engng Sci. 37 (1982), 1419-1421.
[5] Waller, K.V. and T.K. Gustafsson, Fundamental Properties of pH Control, ISA Transactions 22 (1982), No. 1, 25-34.
[6] Gustafsson, T.K. and K.V. Waller, Nonlinear and Adaptive Control of pH, I&EC Research 31 (1992), 2681-2693.
[7] Gustafsson, T.K, An Experimental Study of a Class of Algorithms for Adaptive pH Control, Chem. Engng Sci. 40 (1985), 827-837.
[8] Gustafsson, T.K., B.O. Skrifvars, K.V. Sandström and K V. Waller, Modeling of pH for Control, I&EC Research 34 (1995), 820-827.
[9] Nyström, R.H., K.V. Sandström, T.K. Gustafsson and H.T. Toivonen, Multimodel Robust Control of Nonlinear Plants: A Case Study, J. Process Control 9 (1999), 135-150.